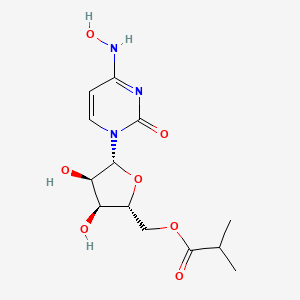
EIDD 2801
| Molecular Formula: | C13H19N3O7 |
|---|---|
| Molecular Weight: | 329.31 g/mol |
[(2R,3S,4R,5R)-3,4-dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl 2-methylpropanoate
UNII YA84KI1VEW
CAS 2349386-89-4
Molnupiravir (development codes MK-4482 and EIDD-2801) is an experimental antiviral drug which is orally active (can be taken orally) and was developed for the treatment of influenza. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication.[1][2] Activity has also been demonstrated against coronaviruses including SARS, MERS and SARS-CoV-2.[3]
The drug was developed at Emory University by the university’s drug innovation company, Drug Innovation Ventures at Emory (DRIVE). It was then acquired by Miami-based company Ridgeback Biotherapeutics, who later partnered with Merck & Co. to develop the drug further.
Safety Controversy
In April 2020, a whistleblower complaint by…
View original post 3,522 more words