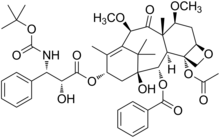
Cabazitaxel
For treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen.
4-acetoxy-2α-benzoyloxy-5β,20-epoxy-1-hydroxy-7β,10β-dimethoxy-9-oxotax-11-en-13α-yl(2R,3S)-3-tert-butoxycarbonylamino-2-hydroxy-3-phenyl-propionate
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(Acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-ene-2-yl benzoate
183133-96-2
Cabazitaxel is prepared by semi-synthesis from 10-deacetylbaccatin III (10-DAB) which is extracted from yew tree needles. The chemical name of cabazitaxel is (2α,5β,7β,10β,13α)-4-acetoxy-13-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1-hydroxy-7,10-dimethoxy-9-oxo-5,20-epoxy-tax-11-en-2-yl benzoate and is marketed as a 1:1 acetone solvate (propan-2-one),
Cabazitaxel is an anti-neoplastic used with the steroid medicine prednisone. Cabazitaxel is used to treat people with prostate cancer that has progressed despite treatment with docetaxel. Cabazitaxel is prepared by semi-synthesis with a precursor extracted from yew needles (10-deacetylbaccatin III). It was approved…
View original post 2,563 more words