Nabriva’s lefamulin receives FDA fast-track status to treat CABP and ABSSS
Austria-based Nabriva Therapeutics has received qualified infectious disease product (QIDP) and fast-track status designation from the US Food and Drug Administration for its lefamulin (BC 3781).
read
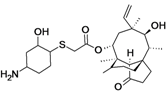
BC-3781
Topical pleuromutilin antibiotic agent
Gram-positive, including MRSA, PHASE 2 COMPLETED,Infection, acute bacterial skin and skin structure (ABSSSI)
Nabriva (Austria), Nabriva Therapeutics AG
BC-3781
cas 1061872-97-6
UNII-61H04Z5F9K
(3aS,4R,5S,6S,8R,9R,9aR,10R)-5-Hydroxy-4,6,9,10-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl [[(1R,2R,4R)-4-amino-2-hydroxycyclohexyl]sulfanyl]acetate;
14-O-[2-[(1R,2R,4R)-4-Amino-2-hydroxycyclohexylsulfanyl]acetyl]mutilin
BC-3781 is a pleuromutilin antibiotic in early clinical development at Nabriva for the treatment of community acquired pneumonia and for the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI). Pleuromutilin antibiotics interfere with bacterial protein synthesis via a specific interaction with the 23S rRNA of the 50S bacterial ribosome subunit. They have a distinct antibacterial profile and show no cross-resistance with any other class of antibiotics. In 2012, a codevelopment agreement was signed between Forest and Nabriva…
View original post 5,321 more words